The voltage-gated potassium (Kv) channels promote the outflow of potassium ions in the repolarization phase of the action potential and play a crucial role in regulating the membrane potential of excitable cells in the neuronal and cardiovascular systems. The human Kv channels are composed of 12 subfamilies (Kv1-Kv12), among which Kv7, also known as KCNQ channels, have important physiological and pathological functions. Dysfunction of KCNQ channels will lead to a number of severe diseases, including seizures, deafness, neurodevelopmental and cognitive disorders, and arrhythmia. Activators or inhibitors for KCNQ channels could be developed as therapeutic drugs for various diseases. However the only drug (Retigabine) targeting KCNQ shows limited efficacy along with certain side effects, suggesting that a better understanding of the mechanism of channel opening will be essential in guiding the new-generation drug discoveries targeting KCNQ channels. Therefore, it was necessary to conduct a study to reveal the structural basis of channel modulation by endogenous ligands and drug candidates so as to guide the future direction in basic biology, translational medicine, and clinical studies.
Recently, Associate Professor Xu Fei from the iHuman Institute together with her collaborator, Professor Shu Yilai from Eye & ENT Hospital of Fudan University, published their research results in a paper entitled “Structural insights into the lipid and ligand regulation of a human neuronal KCNQ channel” in Neuron. This work reported cryo-EM structures of human KCNQ4 channels in the absence and presence of the signaling lipid PIP2 and revealed the mechanism of PIP2 molecules in channel opening. This structural finding provides an accurate template for future targeted drug design and optimization.

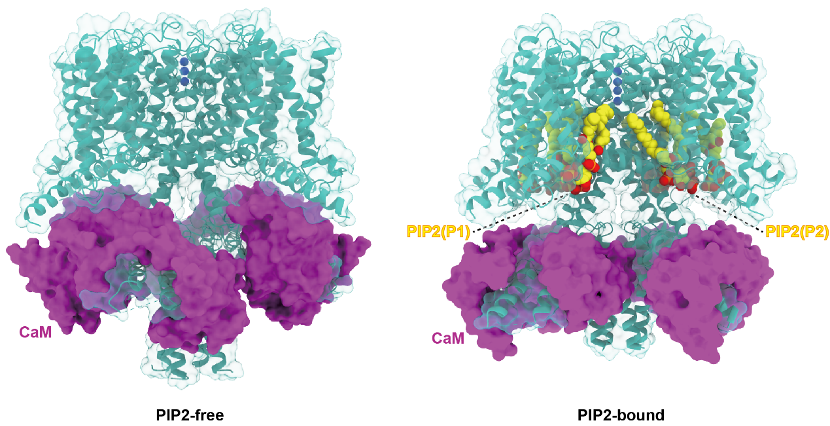
It is known that the channel opening of all KCNQs relies on the signaling lipid molecule PIP2. However, the molecular mechanism of PIP2 in modulating the opening of the four neuronal KCNQ channels (KCNQ2-5), which are essential for regulating neuronal excitability, remains largely elusive. Researchers purified the human KCNQ4 protein and assembled it with PIP2-containing nanodisc to present the target protein in a near-to-native membrane environment. Structural analysis and comparison with the closed-state structure in the absence of PIP2 (also reported in this study) revealed an open conformation of the channel. This is the first report of the open-state structure of a neuronal KCNQ channel thus providing new insight into the field. It is worth mentioning that the structure provides the first-hand validation of this hypothesis, despite a number of biochemical and computational studies in the past that have predicted two potential PIP2 binding sites. Comparing it with the structure of KCNQ4 in the absence of PIP2, the researchers found that binding of PIP2 results in large-scale structural rearrangements occurring in the cytosolic domain and CaM. Moreover, the two PIP2 molecules modulate in a synergistic way in the electromechanical coupling of voltage-sensing domain (VSD) and pore domain (PD) to open the channel. Sequence alignment with other KCNQ channels showed that the two PIP2 binding sites are absolutely conserved, implying a similar modulating mechanism for other KCNQ channels. This study elucidated the molecular mechanism of PIP2 regulating the opening of the KCNQ family of channels, provided new opportunity for drug discovery targeting KCNQ channels, and laid a scientific foundation for the clinical transformation of deafness and other neurological diseases.
Prof. Xu and Prof. Shu are the co-corresponding authors of this paper. The co-first authors include Ph.D. candidates Zheng You and Liu Heng, Ph.D. graduate Dong Shaowei from ShanghaiTech University, and Dr. Chen Yuxin, postdoc of Prof. Shu’s lab.
Link to this paper: https://doi.org/10.1016/j.neuron.2021.10.029

