The research group led by Prof. Xu Fei at iHuman Institute of ShanghaiTech University recently reported a new structure of the Frizzled receptors—an emerging class of cancer targets. They determined the first G-protein complexed Frizzled receptor structure and published their finding on the high-impact journal—Cell research, with a title: “Cryo-EM structure of constitutively active human Frizzled 7 in complex with heterotrimeric Gs”

As the major receptors to the glycoprotein Wnt, Frizzled receptors mediate the Wnt-FZD-βcatenin signaling pathway and play important roles in the human embryonic development and stem cell regulation. Among the ten Frizzleds, FZD7 is the most intensively investigated given its recognized roles in many cancers, including gastric, breast, colorectal and so on. As such, FZD7 is a promising anti-tumor drug target. Our structure of this receptor provides the first-hand information of the precise template for drug design of a new series of therapeutic molecules.
As a class of non-canonical GPCRs, whether the downstream of Frizzled receptors activate the classical G protein pathway has always been the focus of debate in the field. People argued that the Frizzled receptors might not even belong to GPCRs by definition (GPCR is named following its G protein-coupled nature) since there was no structural evidence of their direct coupling to G proteins. Prof. Xu Fei's team creatively assembled the human wild-type FZD7 protein with modified G protein heterotrimer in vitro, and surprisingly identified stable receptor-G protein complex in the absence of any ligand or modulator. With the support of the bio-EM facility of ShanghaiTech University, the research team developed an innovative method using modified 3D cryo-EM reconstruction technology to overcome challenges in data processing and finally obtained the 3.1 angstrom high-resolution structure. Based on the structural analysis, it was confirmed that FZD7 adopted an active conformation, resembling some hallmarks of canonical GPCR activation with distinct features that were unique to the Frizzled receptors. Professor Gunnar Schulte's team from Karolinska Institute in Sweden verified the structural findings through Bio-sensor and other functional methods. This study not only confirmed the existence of classical G protein signaling pathway in Frizzled receptors, but also further demonstrated that FZD7 can constitutively activate downstream G proteins in the absence of ligand, thus providing a new insight for the development of inhibitory drugs targeting this type of receptor.
The PhD graduate student Xu Lu from School of Life Science and Technology, Dr. Chen Bo from iHuman Institute are the two leading authors. Two other co-first authors are Hannes Schihada and Shane C.Wright from Karolinska Institute in Sweden. Other co-authors are from ShanghaiTech University, Karolinska Institute, University of Southern California, and University of Montreal. Prof. Xu Fei, Assistant Director of iHuman Institute and tenured Associate Professor of the School of Life Science and Technology, and Gunnar Schulte, Professor of Karolinska Institute are co-corresponding authors. ShanghaiTech University is the lead affiliation.
Full text link:https://doi.org/10.1038/s41422-021-00525-6
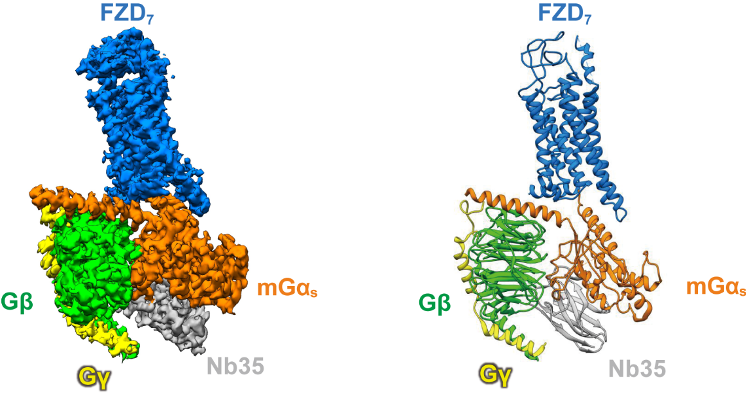
Figure legend:The 3D structure of FZD7 in complex with heterotrimeric Gs protein. (left) the cryo-EM density map; (right) the atomic model.

