A research team from iHuman Institute of ShanghaiTech University has made a new discovery in the field of human chemokine system. This study fills in a critical gap, which is how endogenous chemokines activate endogenous chemokine receptors. The article, entitled "Structure basis of CXC chemokine receptor 2 activation and signaling" is to be published in the prestigious journal Nature, available online as accelerated article preview from July 1, Beijing time.
The first author of the paper is Kaiwen Liu, Ph.D candidate from the School of Life Science and Technology, ShanghaiTech University. The co-corresponding authors are Zhi-Jie Liu, executive director of iHuman Institute, professor of the ShanghaiTech University, and Professor Tian Hua, an independent PI of iHuman Institute. ShanghaiTech is the first affiliation. It is worth noting that Prof. Liu's group has been focusing their research on the structural and mechanistic studies of disease-relevant GPCRs in the past years and this article is their seventh high impact paper.
Figure 1. This chart shows the T cells is migrating towards the cancer cells under the modulation of IL8. In the zoom-in view of T cell surface, the activated chemokine receptor CXCR2 is recruiting downstream G protein (designed by Julie Liu).
There are nearly 50 chemokines and more than 20 chemokine receptors in the human body. Their interaction constitutes a complex chemokine regulatory network that mediates cell migration and is closely related to the inflammation and cancer. The chemokine IL8 activates the G protein-coupled receptor CXCR2 and induce secondary messenger-mediated downstream signaling. CXCR2 mediates migration of neutrophils, T, B and other lymphocytes, and related to a series of biological effects such as degranulation. It plays an important role in inflammation, cell development and chemotaxis of tumor cells. IL8/CXCR2, as a key component of tumor-associated inflammatory environment, can induce chemotactic migration of target cells, promote angiogenesis, and affect the proliferation, survival and metastasis of tumor cells. Therefore, CXCR2 is an important therapeutic target for the treatment of immune diseases and cancer.
The structure and interaction mechanism of IL8 and its receptor CXCR2 can not only reveal the structural model of GPCR regulated by endogenous cytokines, but also contribute to the development of related anti-inflammatory and anti-cancer drugs. Using the Bio-EM facilities of ShanghaiTech, the team led by Prof. Zhi-Jie Liu, successfully determined single-particle cryo-EM structures of CXCR2, bind with two forms of endogenous ligand IL8 and downstream Gi protein with the resolution of 3.5 angstrom (dimer IL8) and 3.4 angstrom (monomer IL8), respectively. The two structures reveal the unique shallow binding pocket and receptor activation mechanism of the endogenous chemokine IL8, as well as the interaction mode and downstream signal transduction mechanism of CXCR2 and Gi protein in the IL8 binding state. "At present, there is little structural information about the interactions between endogenous chemokines and chemokine receptors, which limits the precise design of new anti-cancer drugs. I am very glad to make this breakthrough." Kaiwen Liu said. As the co-corresponding author, Professor Tian Hua added, "In this paper, we report the structures of CXCR2 in inactive and active states. It provides the structure basis of activation mechanism of the chemokine receptors and the design of peptide and antibody drugs."
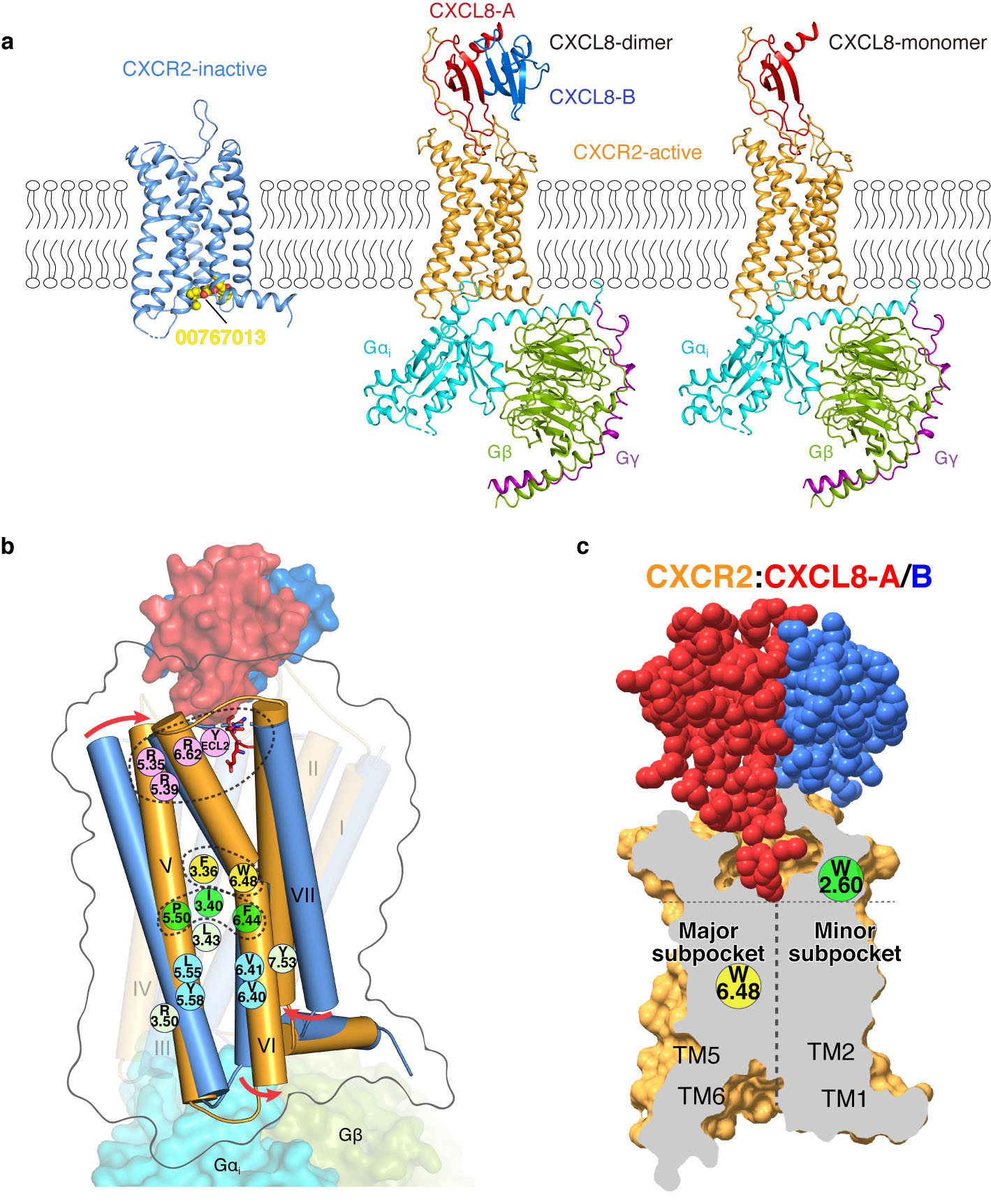
Figure 2. (a) Crystal structure of antagonist 00767013-bound CXCR2 and the cryo-EM structures of CXCR2- CXCL8 (dimer)-Gi and CXCR2- CXCL8 (monomer)-Gi complexes. (b) Schematic summarizing the key translational and rotational movements contributing to CXCR2 activation. (c) Surface cutaway side view comparing ligand binding modes for CXCR2-CXCL8.
"This research is another important breakthrough in our structural and mechanistic study of GPCR signal transductions. We will also carry out the follow-up study on the development of anti-cancer drugs based on the new results." Professor Zhi-Jie Liu said.
A team of collaborators are also involved with this study. They are Prof. Shuguang Yuan from the Shenzhen Institute of Advanced Technology, CAS, Prof. Suwen Zhao from iHuman Institute, and researchers from core facilities of ShanghaiTech University. The research was supported by the Ministry of Science and Technology, the National Natural Science Foundation of China and the Shanghai Municipal Government.
Link: https://www.nature.com/articles/s41586-020-2492-5

Figure 3. Photography of the iHuman Institute at ShanghaiTech University research team. From left to right: Yueming Xu, Suwen Zhao, Tian Hua, Kaiwen Liu, Zhi-Jie Liu, Lijie Wu, Meng Wu, Qianqian Sun.

