Shanghai, May 18, 2017 — An international team led by the Xu lab at the iHuman Institute of ShanghaiTech University and the Amgen Asia R&D Center have determined the crystal structure of the human apelin receptor (APJR) in complex with a 17-aa peptide agonist at 2.6 ?. The results provide a mechanistic understanding of apelin recognition and binding specificity to APJR. The results, described in a paper entitled “Structural basis for apelin control of the human apelin receptor” , is published on May 18, 2017 in Structure, Cell Press.
APJR belongs to class A γ-group of G protein-coupled receptors (GPCRs) recognizing a series of apelin isoforms, such as apelin-36, apelin-17 and apelin-13. Apelin/APJR signaling is involved in diverse (patho)physiological functions such as angiogenesis, vasoconstriction, heart muscle contractility, regulation of energy metabolism, and fluid homeostasis. As such, APJR is an important drug target for various cardiovascular diseases and in particular, heart failure. Yet no APJR-targeting drugs have been approved on market partly due to the lack of structural insight on the molecular basis of ligand recognition. “Visualization of the atomic-resolution ligand binding pocket will uncover this mystery and certainly advance the therapeutic discovery on APJR”, said Fei Xu, Principal Investigator of iHuman Institute and Assistant Professor of School of Life Science and Technology, ShanghaiTech University, and the corresponding author of this paper.
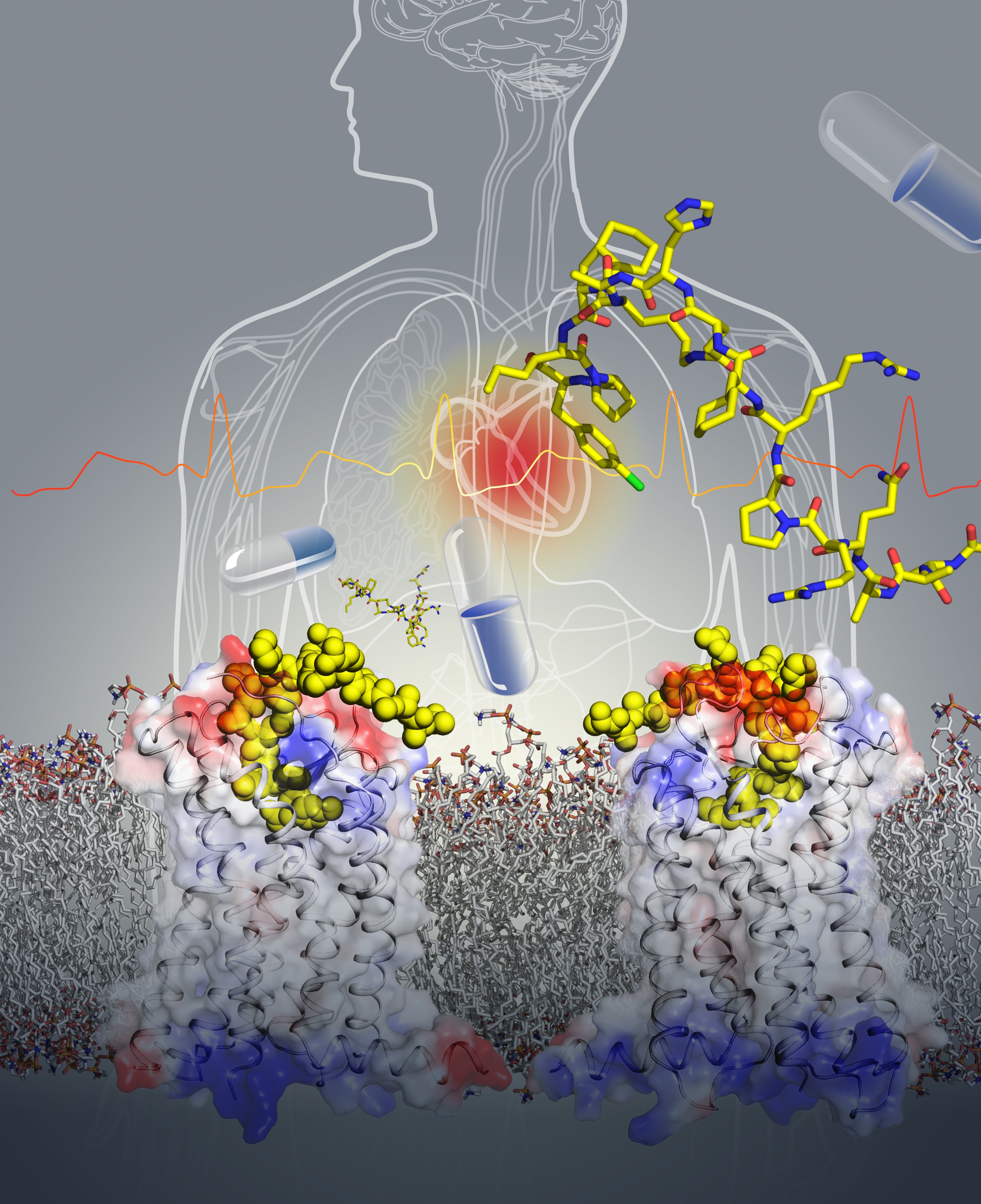
“To stabilize the APJR protein for crystallization, we screened hundreds of mutant constructs and dozens of ligands”, recounted by Dr. Yingli Ma (Amgen), Yue Yang (iHuman), and Dr. Yanbin Ma (Amgen), key authors from both the Xu Lab and the Amgen Asia R&D Center. One of the peptide ligands—AMG3054 that was designed and synthesized by Amgen scientists finally yielded diffraction-quality crystals. AMG3054 is a close mimic of the native apelin-17 peptide with a lactam ring constrained curved structure. The APJR-AMG3054 complex structure reveals a unique two-site ligand binding mode that is not seen in any other reported class-A peptide GPCR structures before. Molecular dynamics simulations and mutagenesis studies validate that such a two-site binding mode likely also holds true for the endogenous ligand, the apelin peptide. Comparison of this structure to other peptide receptors suggests that the two site binding mechanism may be general to endogenous peptide ligand binding. “Drug discovery of GPCRs that recognize endogenous peptides like apelin and apela will greatly be enabled by atomic resolution structural characterization given the more complex binding landscape and open binding surfaces”, said Professor Raymond Stevens, Director of iHuman Institute, ShanghaiTech University.
This work is a successful collaboration between iHuman institute and the Amgen Asia R&D center through the GPCR consortium. “Both the iHuman team and the Amgen team took full advantage of Shanghai co-localization with each other and engaged in frequent scientific discussions. Our joint success in solving a highly impactful GPCR structure within a short period of time is a win-win situation and sets an excellent example for academic-industry collaborations”, commented by Dr. Mingqiang Zhang, Vice President of Research and Head of the Amgen Asia R&D Center. Other significant contributors to this paper are Qingtong Zhou, Suwen Zhao, Chao Li from ShanghaiTech University, and scientists from Amgen Asia R&D center. Financial support to this work comes from, in part, Shanghai Municipal Government, ShanghaiTech University, Shanghai “Pujiang Talents” grant, and the GPCR Consortium.
Article link: http://www.sciencedirect.com/science/article/pii/S0969212617301260
Artistic illustration of the APJR-AMG3054 structure. Other background art includes the human body, heart, and drug capsules hinting at the cardiovascular therapeutic impact from the structure determination. Image courtesy of Yekaterina Kadyshevskaya, Stevens Laboratory.
iHuman team from left to right: Qingtong Zhou, Suwen Zhao, Xu Fei, Yue Yang, and Li Chao.